교육신청
- 전체
- PI,Sub-I
- IRB(의사)
- IRB(의사 외)
- CRP
- CRA
- CRC
- QA
- GCP
- 공통
- 중개임상
- 임상시험설계
- PM
- 규제과학,인허가
- DM
- 통계
- MW
- PV
- 기초의학이론
- 임상시험 품질관리
- 대학연계프로그램
- KIC
[규제과학,인허가 > 보수]
[KoNECT-Premier Research] Regulatory Symposia
소개
목차
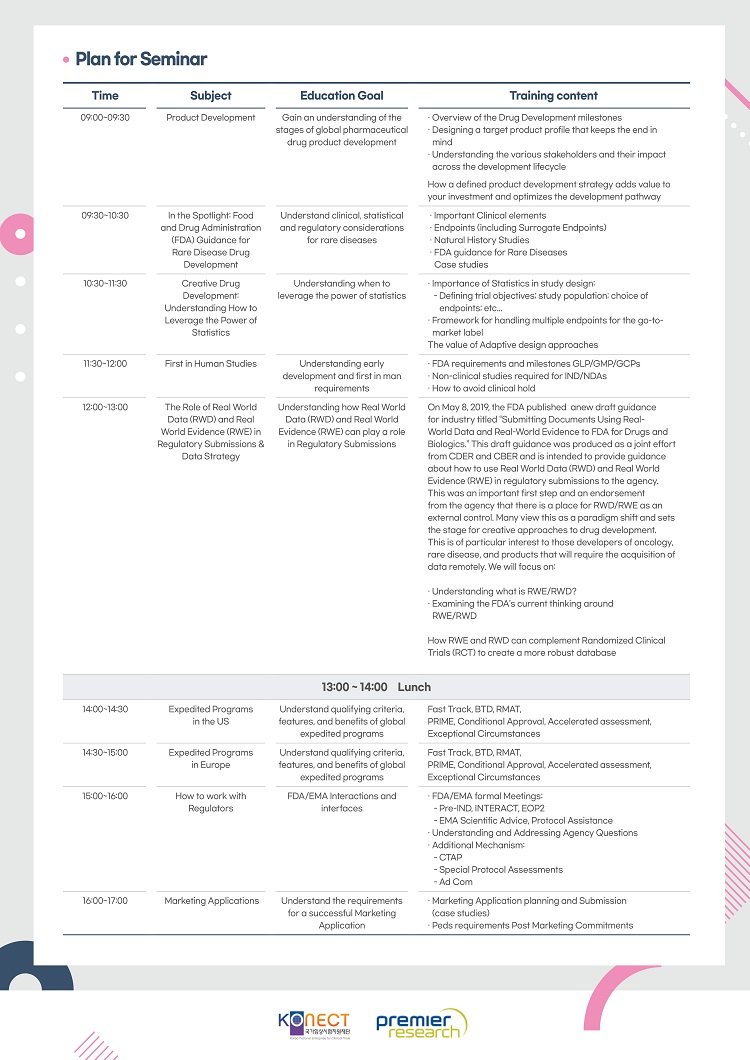
교육일정
학습개요

교육내용
참고사항
*해당 교육은 모두 영어로 진행됨을 알려드립니다.
*본 강의는 임상시험종사자 교육으로 인정되지 않습니다.
*신청자가 많은 경우 기관별로 신청인원수를 제한할 수 있습니다.
수료/과락 기준
| 평가기준 | 집합 출석율 | 온라인 진도율 | 시험 | 과제 | 토론 | 기타 |
|---|---|---|---|---|---|---|
| 배점비율 | 100.00% | - | - | - | - | - |
| 과락기준 | 80% | - | - | - | - | - |

