| No. |
Classification |
Name of Lecture |
Study period |
Hours |
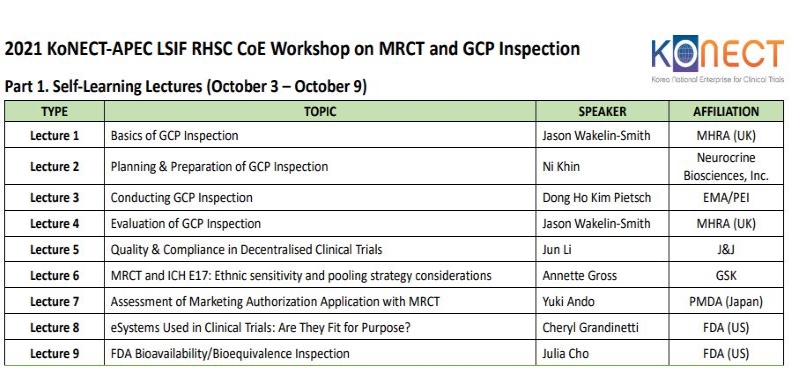
| 1 |
Online/Lecture |
Basics of GCP Inspection |
2021-10-03 09:30 ~ 2021-10-24 23:50
|
43minute
|
| 2 |
Online/Lecture |
Planning & Preparation of GCP Inspection |
2021-10-03 09:30 ~ 2021-10-24 23:50
|
1hours
14minute
|
| 3 |
Online/Lecture |
Conducting GCP Inspection |
2021-10-03 09:30 ~ 2021-10-24 23:50
|
53minute
|
| 4 |
Online/Lecture |
Evaluation of GCP Inspection |
2021-10-03 09:30 ~ 2021-10-24 23:50
|
46minute
|
| 5 |
Online/Lecture |
Quality & Compliance in Decentralised Clinical Trials |
2021-10-03 09:30 ~ 2021-10-24 23:50
|
56minute
|
| 6 |
Online/Lecture |
MRCT and ICH E17: Ethnic sensitivity and pooling strategy considerations |
2021-10-03 09:30 ~ 2021-10-24 23:50
|
52minute
|
| 7 |
Online/Lecture |
Assessment of Marketing Authorization Application with MRCT |
2021-10-03 09:30 ~ 2021-10-24 23:50
|
46minute
|
| 8 |
Online/Lecture |
eSystems Used in Clinical Trials: Are They Fit for Purpose? |
2021-10-03 09:30 ~ 2021-10-24 23:50
|
32minute
|
| 9 |
Online/Lecture |
FDA Bioavailability/Bioequivalence Inspection |
2021-10-03 09:30 ~ 2021-10-24 23:50
|
44minute
|
| 10 |
Online/Questionnaire |
Multi-Regional Clinical Trials(MRCT) and Good Clinical Practice(GCP) Inspection_Survey |
2021-10-03 09:00 ~ 2021-10-31 23:50
|
-
|
 ?
?